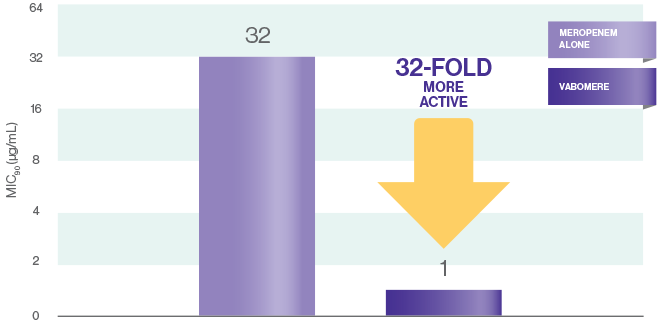
VABOMERE® has 32-fold more in vitro activity than meropenem alone against KPC-producing Enterobacterales1*
Adding vaborbactam significantly reduces the MIC of meropenem alone against KPC-producing Enterobacterales1
-
Meropenem alone and in combination with vaborbactam was evaluated against KPC-producing strains of
Enterobacterales involving
>1900 isolates1
MIC90 FOR MEROPENEM VS VABOMERE® (MEROPENEM AND VABORBACTAM)1†
*In vitro activity does not necessarily correlate with clinical efficacy.
†Study description
The activity of meropenem in combination with vaborbactam against >1900 KPC-producing clinical
isolates of Enterobacterales was evaluated in both prospective and retrospective in vitro studies.
Vaborbactam potentiated the activity of meropenem in these studies; lowest concentrations of the
antibiotic at which 90% of the isolates were inhibited (MIC90s) were >32 μg/mL for
meropenem alone and were reduced to 0.5-
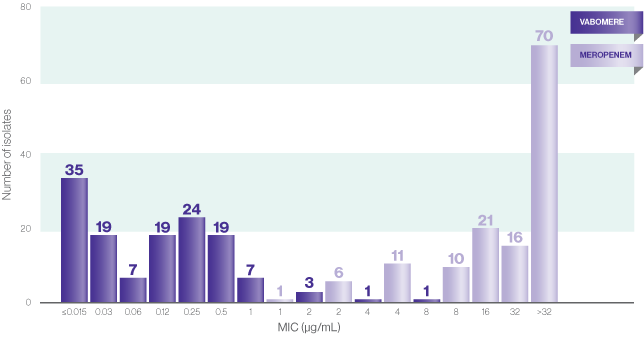
VABOMERE restored activity of meropenem against select KPC-producing isolates2
Over 90% of the 135 KPC-producing isolates had an MIC <1 μg/mL2*
MICs OF VABOMERE AND MEROPENEM2,3*
*In vitro activity does not necessarily correlate with clinical efficacy.
- 135 KPC-producing Enterobacterales clinical isolates were evaluated, in a study of >14,000 gram-negative clinical isolates, from 82 hospitals worldwide in 20142
- A total of 135 (50.9% of the CRE isolates; 1.3% of the overall population) isolates carried blaKPC genes, including 60 blaKPC-2, 74 blaKPC-3, and 1 blaKPC-4 gene2
- In this study, 133 of the 135 organisms of the KPC-producing isolates were inhibited by meropenem-vaborbactam at ≤2 μg/mL, and all isolates were inhibited by this combination at ≤8μg/mL2
Study description
A total of 14,304 gram-negative bacterial clinical isolates were consecutively collected in 82 worldwide hospitals. All isolates were tested for susceptibility against meropenem-vaborbactam and comparators using the reference broth microdilution method as described by the Clinical and Laboratory Standards Institute (CLSI). Meropenem was combined with vaborbactam at a fixed concentration of 8μg/mL.2
VABOMERE addresses a broad spectrum of resistant gram-negative pathogens4,5
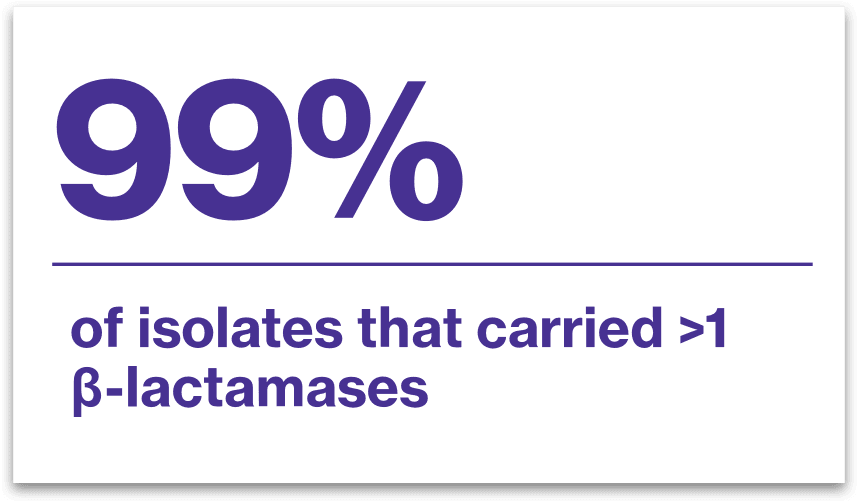
Vaborbactam inhibits a number of β-lactamases and ESBLs4
- VABOMERE demonstrated in vitro activity against Enterobacterales in the single or multiple β-lactamases from various classes (KPC, SME, TEM, SHV, CTX-M, CMY, and ACT groups)4*
VABOMERE RESTORES THE POTENCY OF MEROPENEM1†
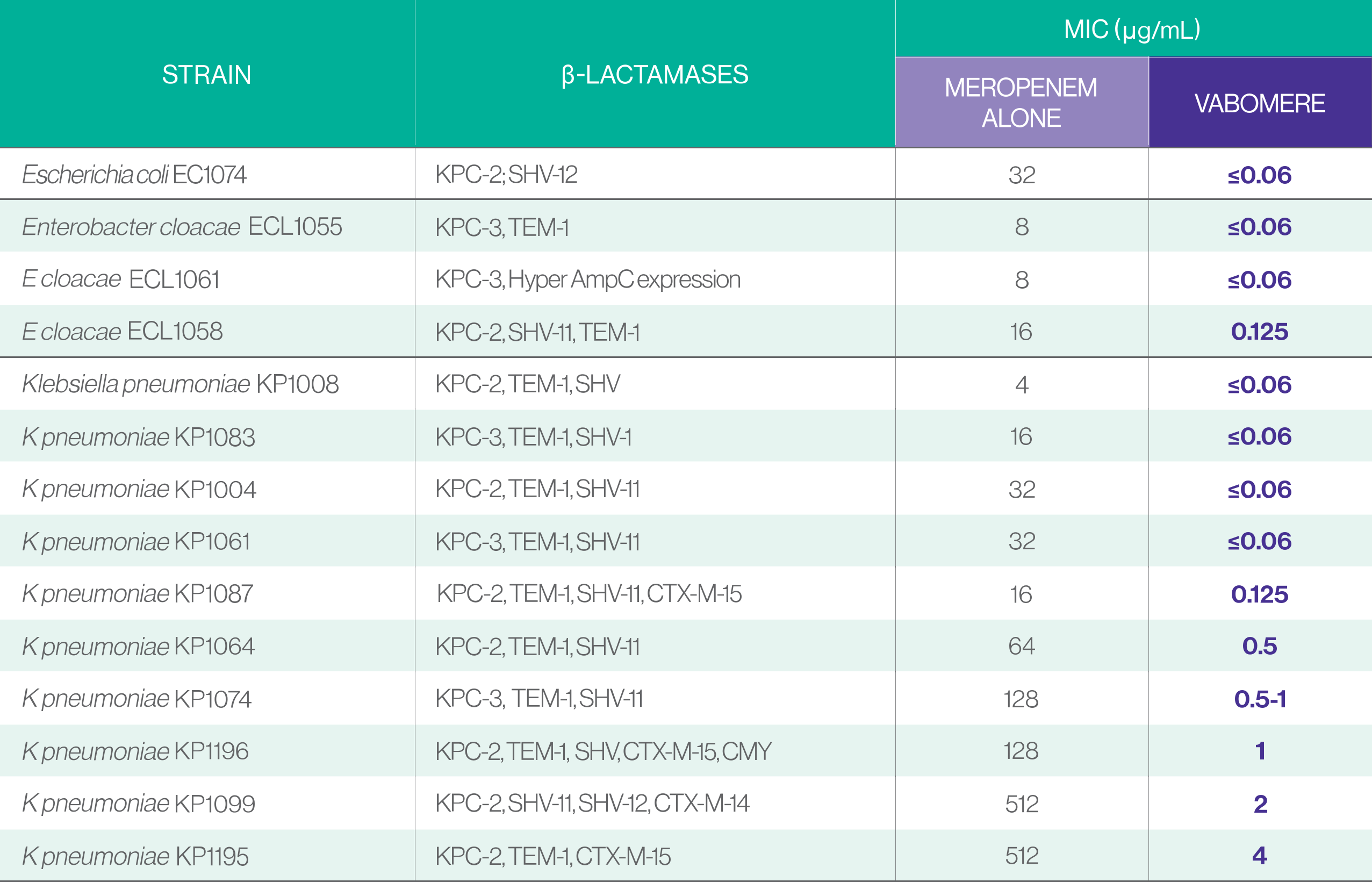
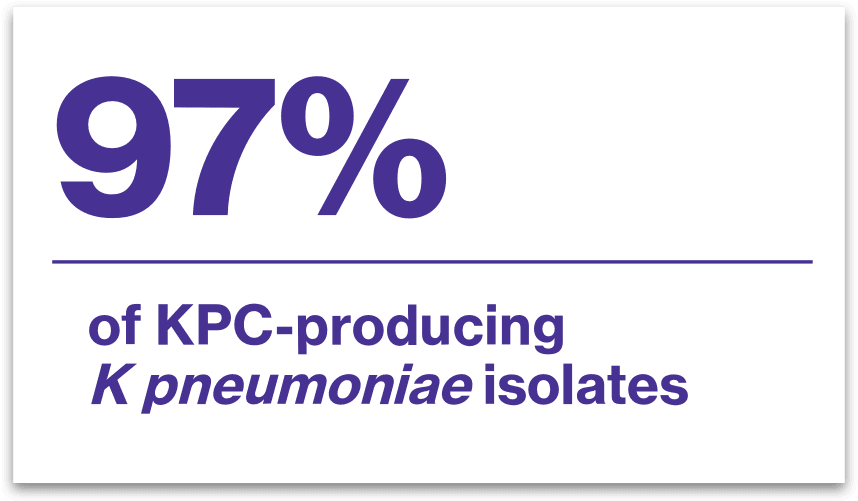
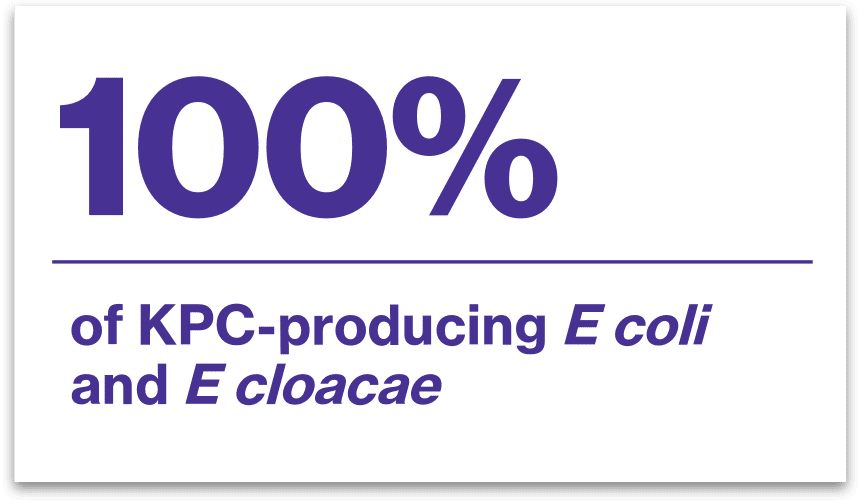
VABOMERE inhibited2*:
VABOMERE demonstrated in vitro activity against additional gram-negative bacteria including4:
- Citrobacter freundii
- Citrobacter koseri
- Enterobacter aerogenes
- Klebsiella oxytoca
- Morganella morganii
- Proteus mirabilis
- Providencia spp.
- Pseudomonas aeruginosa
- Serratia marcescens
The efficacy of VABOMERE in treating clinical infections due to these bacteria has not been established in adequate and well-controlled clinical trials, and in vitro data does not necessarily correlate to clinical efficacy.