How to order VABOMERE® for your institution
Order VABOMERE® (meropenem and vaborbactam) using standard ordering procedure through your specialty distributor
- VABOMERE is a specialty distributor stocked product
- Customers with specialty accounts can continue to order until 7pm ET and receive product the next business day. For special circumstances, you can receive an emergency order on a Saturday or Sunday. Please check with your specialty distributor for details
- Download the Order Sheet for more information
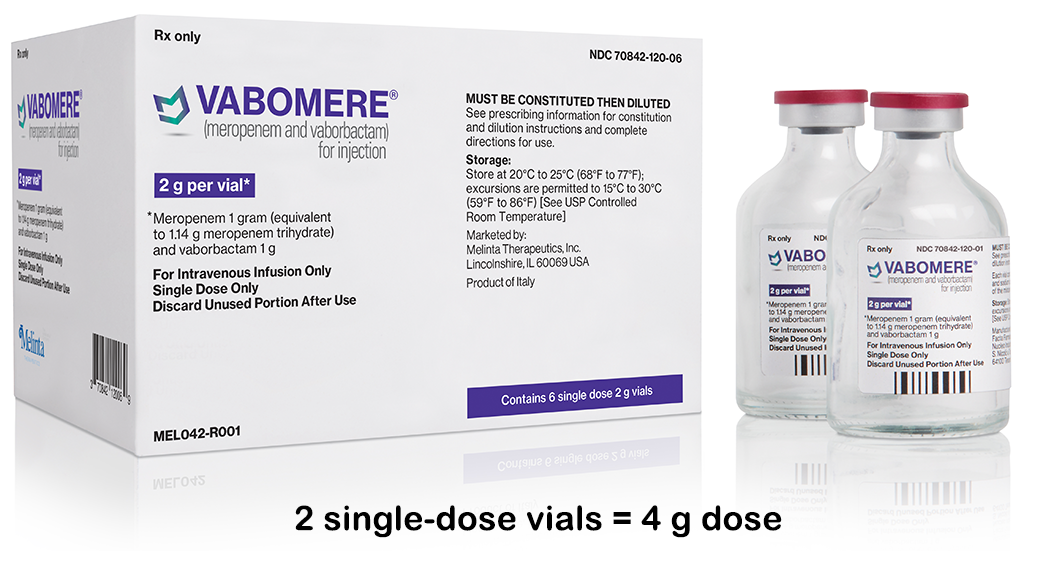
VABOMERE comes in a box of 6 vials, the typical course of daily treatment*
How it’s supplied1:
VABOMERE is supplied as a white to light yellow sterile powder for constitution in single-dose, clear glass vials that contain 2 grams of VABOMERE. Each vial contains 1 gram of meropenem (equivalent to 1.14 grams of meropenem trihydrate), 1 gram of vaborbactam, and 0.575 gram of sodium carbonate.
ONE BOX is ONE DAY of treatment.*
VABOMERE 4 g (2 g meropenem and 2 g vaborbactam)
NDC NO.
70842‑0120‑06
Each vial contains VABOMERE 2 g (1 g meropenem and 1 g vaborbactam) for injection and is supplied in a box of 6 vials.
Typical full course of daily treatment for patients with eGFR ≥50 mL/min/1.73 m2. Additional dosing information for patients with renal impairment can be found in the Dosing section of this website.
VABOMERE Specialty Distributor Ordering Information:
| Distributor Name | Customer Service Phone | Customer Service Email |
|---|---|---|
| AmerisourceBergen Specialty Distribution | 800-746-6273 | Asd.customerservice@asdhealthcare.com |
| Cardinal Health Specialty Pharmaceutical Distribution | 855-855-0708 | https://www.cardinalhealth.com/en/solutions/specialty-distribution-services.html |
| Curascript Customer Service | 877-599-7748 | Customer.Service@curascript.com |
| McKesson Plasma and Biologics | 877-625-2566 | mpborders@mckesson.com |
| McKesson Specialty Health | 855-477-9800 | mshcustomercare-mspl@mckesson.com |
| McKesson Specialty Health (oncology customers) | 800-482-6700 | oncologycustomersupport@mckesson.com |
| Morris & Dickson | 800-388-3833 | customerservice@morrisdickson.com |
Billing and coding information for VABOMERE
Covers the coding requirements, coding systems, and their application when VABOMERE is administered
-
Lists ICD-10 codes and transition of care codes to facilitate timely claims processing and reduce
the risk of denied claims
–Includes the permanent J Code (J2186) for VABOMERE - Addresses coverage and payment information pertaining to Medicare, Medicaid, and commercial payers, in inpatient and outpatient settings, including long-term acute care and home infusion reimbursement
- Includes sample CMS 1450 and CMS 1500 billing forms
- See Coding and Billing Reference Guide for detail